|
|
|
|
News The Project Technology RoboSpatium Contribute Subject index Download Responses Games Gadgets Contact <<< Redox reaction Electrode potential >>> ElectrolysisDissociationWhen small grains of sand and water are stirred together, the water starts to become murky. When the stirring stops, the process of sedimentation starts, meaning the grains settle out of the fluid coming to rest at the bottom of the pot and the water becomes clear again. You still can see the grains of sand at the bottom of the pot. If you repeat this experiment using grains of sodium chloride instead of sand, the water is murky just for a small span of time. After stopping the stirring, the grains of sodium chloride seem to have disappeared and even after a long span of time no grains can be seen at the bottom of the pot. Sodium chloride is an ionic compound of positively charged sodium cations and negatively charged chloride anions with the formula NaCl. Water consists of molecules with the formula H2O: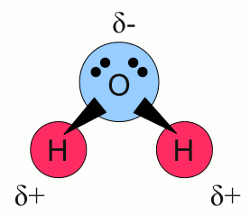 Water molecule:
Water molecule:Water molecules consists of one oxygen atom forming covalent bonds with two hydrogen atoms. The two bonds are not arranged in a linear way but form an angle of about 104 degrees, with the hydrogen atoms at the tips and the oxygen atom at the vertex. Furthermore oxygen has a higher electronegativity than hydrogen resulting in a non-uniform distribution of the electron pairs forming the chemical bonds. At the drawing this fact is visualized by triangles instead of lines between the atoms. For this reason the side of the molecule where the oxygen atom is placed, has a partial negative charge and the tips with the hydrogen atoms have a partial positive charge. Molecules with a charge difference like this are called dipoles. The relatively positive areas of a water molecule are attracted by the relatively negative charged area of other water molecules or by charged particles like ions. When an ionic compound enters water, the partially negative dipole end is attracted by the cations, the partially positive dipole ends are attracted by the anions. The small size of the water molecules allows them to surround the ions. During this process of hydration, an ionic compound like sodium chloride is separated into Na+ cations and Cl- anions which are transported away from their crystal lattice. Sodium chloride and water form an aqueous solution with water functioning as the solvent and sodium chloride as solute. The process of separating the ions from their crystal lattice is called dissociation. ElectrolyteA substance containing free ions is called an electrolyte. When cupric chloride (CuCl2) is placed in water, the crystalline salt starts dissociating, resulting in an aqueous solution of Cu2+ and Cl- ions moving freely between the water molecules. What happens, if this solution is exposed to an electric field? Well, at the chapter about voltage we have learned that a force is acting on charged particles inside an electric field. Some later at the chapter about current we saw that charged particles start moving when being exposed to an electric field (as far as they aren't fixed). Those moving charged particles or the rate of flow is called current. We can expose the aquatic solution of cupric chloride to an electric field by placing two plates of conducting material at two sides of the pot and connecting the plates to a voltage source: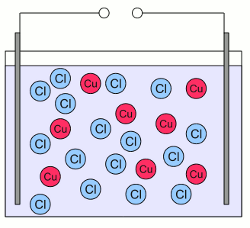 Aqueous solution of cupric chloride:
Aqueous solution of cupric chloride:The solid crystal lattice of cupric chloride is dissociated into single, free Cu2+ cations (red circles) and the double number of Cl- anions (blue circles). Cations and anions are arranged homogeneously inside of the pot. 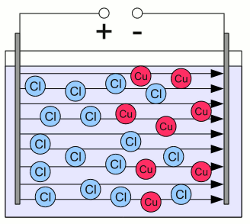 The aqueous solution is exposed to an electric field by using two plates of conducting material. The left plate is connected to the positive terminal of a voltage source and the right plate to the negative terminal. An electric field is created, similar to those of a capacitor. As soon as the electric field is established, forces are acting on the charged ions. The positively charged cations are pulled to the negatively charged plate, the negatively charged anions are pulled to the positively charged plate. As a result of the movement, the ions are arranged inhomogeneous which leads to the creation of an electric field, too. The electric field created by the ions weakens those of the external voltage source. In contrast to electrons, which could enter the plates, the movement of the ions stops when they touch the plates. As soon as the potential of the electric field, created by the ions has reduced those of the external voltage source to zero, the force acting on the charged particles and so the movement of the ions stops.
The aqueous solution is exposed to an electric field by using two plates of conducting material. The left plate is connected to the positive terminal of a voltage source and the right plate to the negative terminal. An electric field is created, similar to those of a capacitor. As soon as the electric field is established, forces are acting on the charged ions. The positively charged cations are pulled to the negatively charged plate, the negatively charged anions are pulled to the positively charged plate. As a result of the movement, the ions are arranged inhomogeneous which leads to the creation of an electric field, too. The electric field created by the ions weakens those of the external voltage source. In contrast to electrons, which could enter the plates, the movement of the ions stops when they touch the plates. As soon as the potential of the electric field, created by the ions has reduced those of the external voltage source to zero, the force acting on the charged particles and so the movement of the ions stops.The conductor plates are called electrodes. The electrode connected to the negative terminal of the voltage source is called cathode, those being connected to the positive terminal is called anode. In an electrolyte, positively charged ions (cations) are attracted by the negatively charged cathode and negatively charged ions (anions) are attracted by the positively charged anode. Redox reactionLike mentioned above, the ions of an electrolyte can't enter the electrodes. When the ions get in contact with one of the electrodes, chemical reactions are initiated. At the positively charged electrode, electrons are leaving the electrolyte when an anion gets in contact with the anode. The anion is loosing one or more electron(s) which leads to an increase of it's oxidation state thus it is oxidized. At the negatively charged electrode (cathode), the cations gain electrons which leads to a decrease of their oxidation state, thus the cations are reduced. Applied to the example above, the following chemical processes are initiated at the electrodes:Positively charged copper ions are reduced to elemental copper atoms at the cathode: Negatively charged chloride ions are oxidized to chlorine molecules at the anode: The electrons transferred from the anions to the anode are leaving the electrolyte. Subsequently the are "pumped" to the cathode by the voltage source. There the electrons enter the electrolyte when being transferred to the cations, hence the electrical circuit is closed and a permanent current is running through the electrolyte. This process is called electrolysis. The conductivity of an electrolyte depends on the number and the mobility of the dissociated ions and their charge. While a current is running through an electrolyte, the ions that gain or lose electrons to become uncharged atoms, separate from the electrolyte, hence the number of ions and for this reason the conductivity decreases over time. <<< Redox reaction Electrode potential >>> News The Project Technology RoboSpatium Contribute Subject index Archives Download Responses Games Links Gadgets Contact Imprint |
|
|